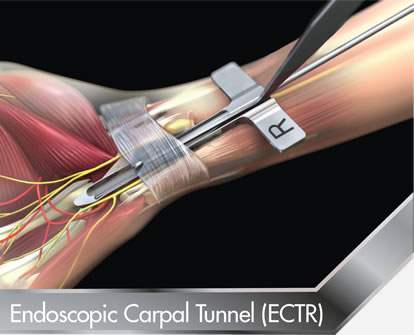
Endoscopic Carpal Tunnel Release (ECTR)
S.E.G-WAY™ is the first Endoscopic Carpal Tunnel (ECTR) system designed to properly position the incision in the Ulnar safe Zone. It is also the only system that is anatomy and patient specific. It provides a level of safety and quality that is unavailable using any other method.The Endoscopic Carpal Tunnel (ECTR) system provide the surgeon with the ability to rasp and probe before and after the release for enhanced visualization and safety.
FEATURES AND BENEFITS
| SAFETY FIRST | ANATOMY SPECIFIC left and right guides place the blade in the Ulnar Zone LARGER FIELD OF VIEW a wider, longer, panoramic field of view RASP to visualize transferse fibers before release |
| VERSATILITY | PATIENT SPECIFIC different size guides for smaller and larger hands |
| ASSURANCE | VISUALLY PROBE the distal end before release |
| CONFIDENCE | PROBE uncut fibers to ensure a complete release |
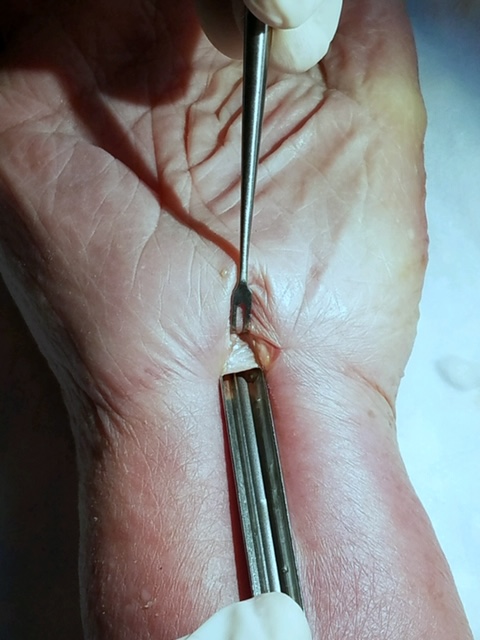
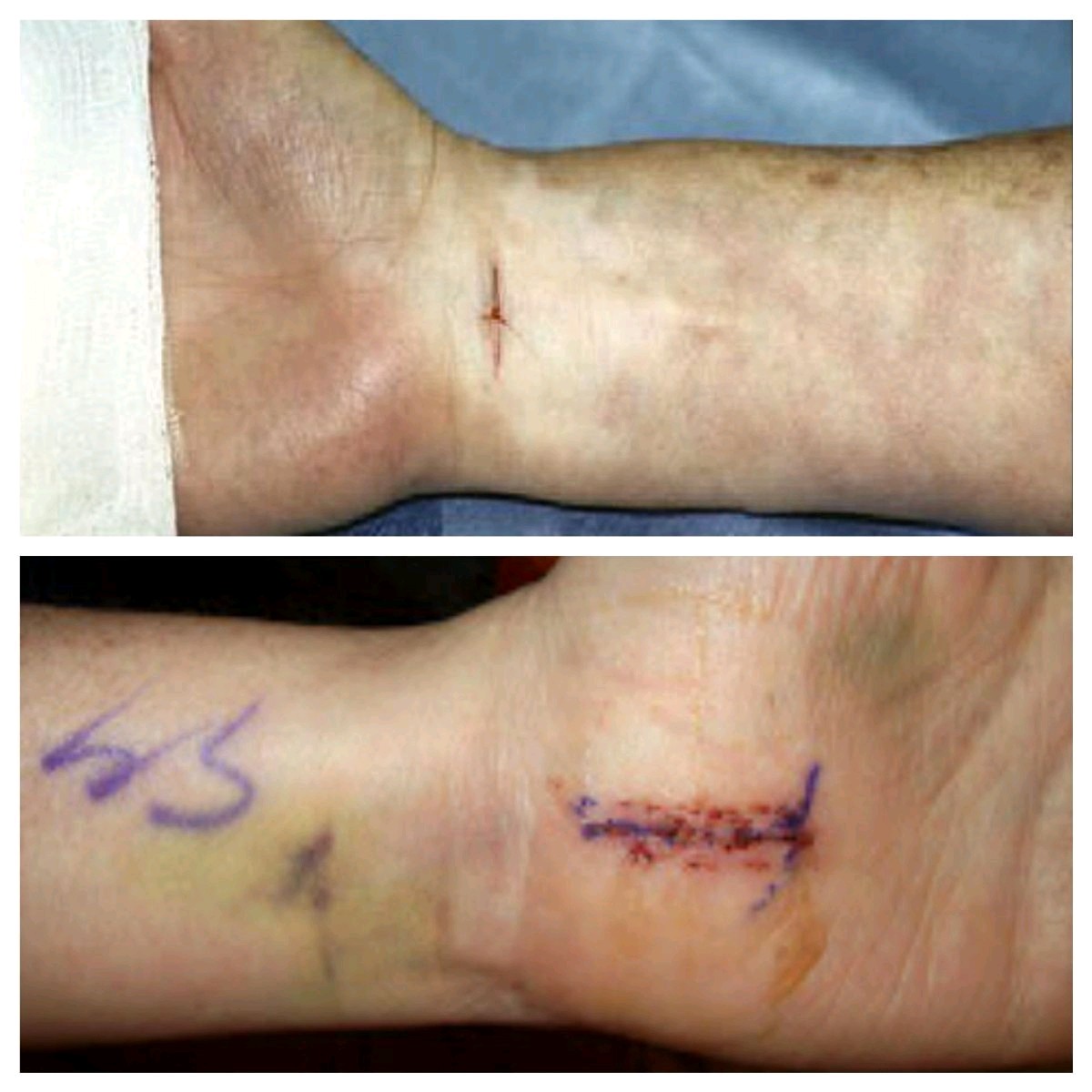
| UNIPORTAL | SMALLER incision in the wrist |
ECTR Surgical Technique
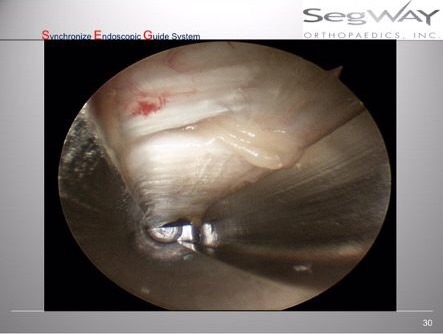
ECTR Field of View
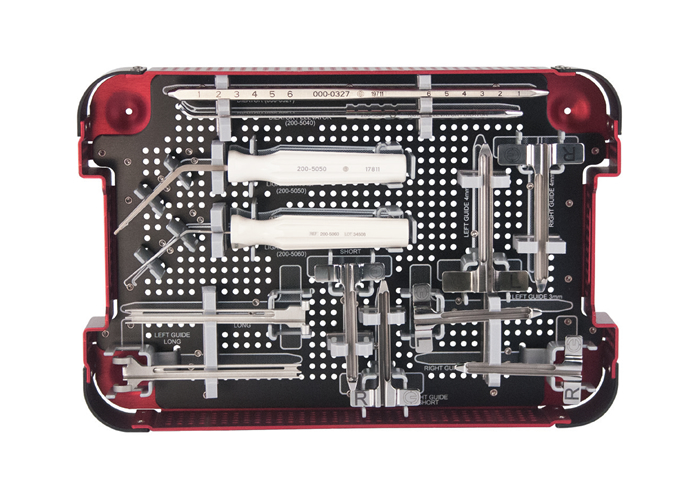
PRODUCT LINE INFORMATION
| Description | Part# |
| S.E.G-WAY™ Instrument Set (Complete Set) | 200-5560 |
| Left Medium SS Knife Guide (4mm) | 200-5012 |
| Right Medium SS Knife Guide (4mm) | 200-5022 |
| Left Small SS Knife Guide (2.7mm) | 200-5014 |
| Right Small SS Knife Guide (2.7mm) | 200-5024 |
| Left Large SS Knife Guide (4mm) | 200-5010 |
| Upper ECuTR Guide (4.0mm long) | 200-6041 |
| Lower ECuTR Guide (4.0mm long) | 200-6042 |
| Synovial Dilator / Elevator | 200-5040 |
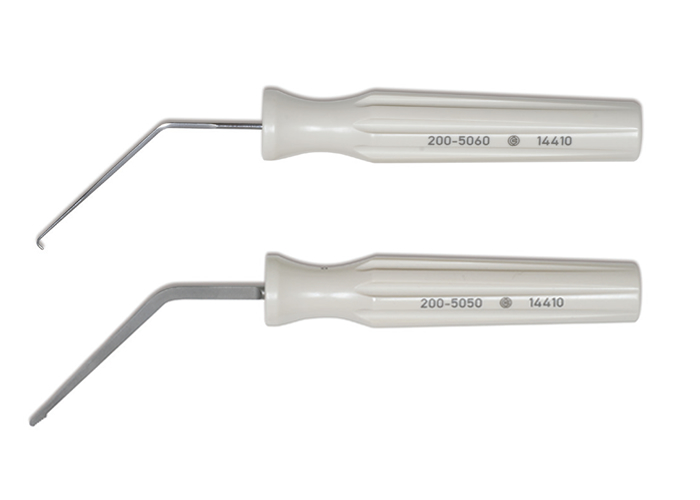
| Rasp, Ligament | 200-5050 |
| Probe, Ligament | 200-5060 |
| Dilator | 000-0327 |
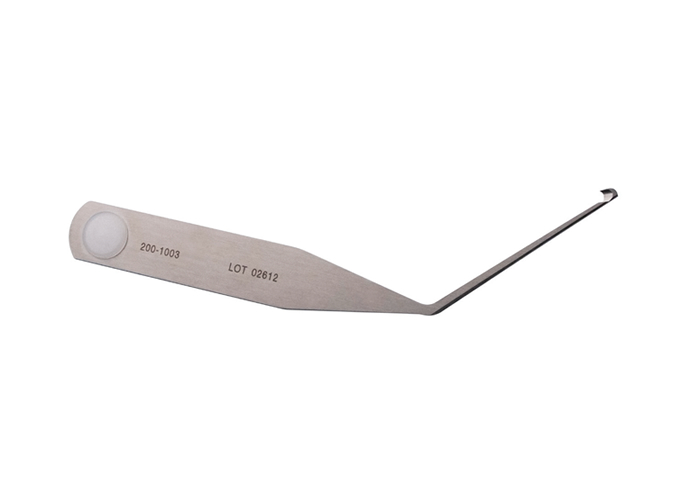
| Retrograde Ligament Knife (SINGLE USE) | 200-1003 |
| Antegrade Ligament Knife (SINGLE USE) | 200-1006 |